Zinc coatings – acid or alkaline process
For decades, zinc coating has been established and used globally as a good means of protecting steel in environments subject to mild to moderate corrosion.
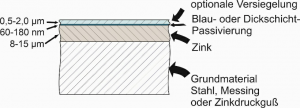
During zinc electroplating, a layer of usually between 8 and 15 micrometres of zinc is deposited at an electrolyte temperature of approx. 30°C. In the acid zinc process, a thicker layer is often used.
To increase the corrosion protection, various passivation layers are then applied to the zinc: e.g. the slightly iridescent thick-layer passivation with a thickness of approx. 140 to 180 nanometres or blue chromate passivation, with a thickness of around 40-60 nanometres.
The components can then be provided with a silicate-based or polymer-containing sealer to further improve scratch resistance and corrosion protection.
This results in a long-lasting coating that delivers precisely tailored corrosion protection at a reasonable price.
Thanks to the good distribution of layer thickness, depending on the process, features such as threads are no problem.
Corrosion protection standards:
- Up to 168 h without zinc corrosion
- up to 360 h without base material corrosion in a neutral salt spray test
Examples:
- DIN 19598 – Fe//Zn8-15/Cn//T2
- DIN 19598 – Fe//Zn8-15/An//T0
- Daimler Benz: DBL 8451.16
- MAN: 183-3 B13
- Continental: PP212
- VW: 13750 r342/r343
- Parker: FC-F01
- Liebherr: LN 252-7