Zinc-iron coatings – alkaline process
Zinc-iron coating has been established since the 1990s as a means of high-quality corrosion protection for steel and is primarily used in automotive manufacturing.
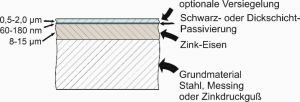
During zinc electroplating, between 0.3% and 0.9% iron is electrolytically incorporated into the coating as an alloying element at an electrolyte temperature of approx. 25°C.
To increase the corrosion protection, a slightly yellowy iridescent or a black passivation layer is then applied to the zinc-iron, with a thickness of approx. 140 to 180 nanometres.
The components can then be provided with a silicate-based or polymer-containing sealer to further improve scratch resistance and corrosion protection. (This is essential in the case of black passivation).
Silicate-based sealers withstand aggressive chemicals such as hydraulic oils and brake fluids.
For their part, polymer-containing sealers reduce the coefficient of friction and improve characteristics such as scratch resistance and screwing behaviour.
This results in a long-lasting coating that exceeds the corrosion protection of conventional zinc coatings six-fold – with the same thickness (e.g. 8-12 micrometres).
The excellent distribution of layer thickness ensures that requirements such as tight-tolerance fits are no problem.
Corrosion protection standards:
- Up to 360 h without zinc corrosion
- Up to 600 h without base material corrosion in a neutral salt spray test
Examples:
- Daimler Benz: DBL 8451.86
- BMW: GS 90010 ZNFE SI
- DIN 19598 – Fe//ZnFe8-15/Cn//T2
- VW: 13750 r301 / r302
- MAN: 183-3 B4
- Liebherrr: TLV 12250